What GLP-1 drugs reveal about MPNs, inflammation, and cancer risk

A surprising link between inflammation, clonal blood stem cells, and a blockbuster drug opens new questions in blood cancer research.
In recent years, glucagon-like peptide-1 receptor agonists (GLP-1RAs) — the class of drugs that includes Ozempic, Wegovy, and others — transformed care for type 2 diabetes and obesity.
Now, new research from Cleveland Clinic physician-scientist Dr. Abhay Singh suggests these same drugs may do more than regulate metabolism — they may influence the risk of rare, inflammation-driven blood cancers like myeloproliferative neoplasms (MPNs) and myelodysplastic syndromes (MDSs).
What GLP-1 drugs are and what else they might do
GLP-1 receptor agonists (GLP-1RAs) have seen a meteoric rise in popularity since the first drug in this class, exenatide, was approved by the FDA in 2005. These therapies are now widely used for both type 2 diabetes and weight management. By activating GLP-1 receptors found in the intestine, brain, heart, and other tissues, GLP-1RAs can:
- help regulate blood sugar
- protect the cells responsible for producing insulin
- slow stomach emptying
- promote feelings of fullness
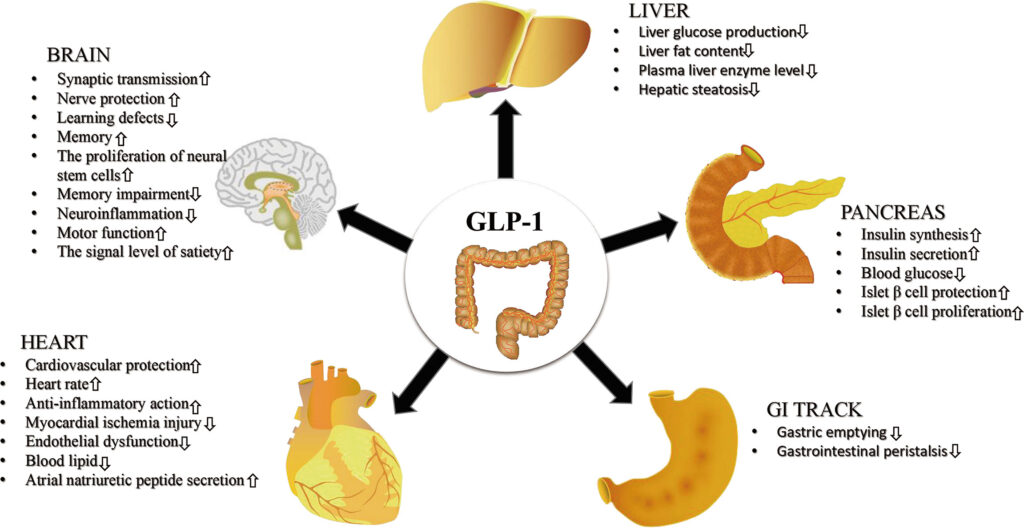
Caption: GLP-1RAs have direct and indirect effects on a diverse group of tissues and cell types, increasing or decreasing their activity. Originally published in Frontiers in Endocrinology, Zhao et al., 2021
Although GLP-1RAs act on multiple tissues throughout the body, the exact mechanisms behind their wide-ranging effects remain unclear. One especially intriguing outcome, however, has caught researchers’ attention: “They decrease inflammation by unknown mechanisms,” Dr. Singh. says, then laughs. “If anyone would say they know the mechanism, they would be making a lot of things up!” Singh and others in the field have seen patterns worth pursuing. “Your weight goes down, the chronic state of inflammation or unchecked inflammation improves.”
That matters, especially in cancers like MPNs and MDSs, where inflammation fuels the growth of harmful clones of blood-forming cells. Singh’s new study sets out to explore whether patients on GLP-1RAs might experience lower rates of these inflammation-linked blood cancers.
What the study found
Unlike many traditional stories of discovery, this exploration starts big, with population findings. In their retrospective study, Singh and first author Omer Ashruf, along with their Cleveland Clinic colleagues, analyzed health records from one of the largest health datasets available. They compared hematologic cancer rates in people with type 2 diabetes treated with insulin, metformin, or GLP-1RAs. The numbers were large: about 1 million patients on insulin, 500,000 on metformin, and 50,000 on GLP-1 drugs.
Across the board, GLP-1 drugs were associated with a lower risk of hematologic cancers compared to insulin. But the most surprising result came when they looked at MPNs and MDSs specifically.
The study compared the health outcomes of patients treated with the well-document oncoprotective drug metaformin to GLP-1 receptor agonists. One might expect comparable or slightly different effects. Instead, while metformin did not affect the MDS or MPN rates, “In our study, GLP-1RAs demonstrated a statistically significant superiority in their association with reduced risk of MDS and MPNs,” Singh says. That difference hints at something unique about GLP-1RA’s unknown mechanisms.
Why MPNs and MDSs stand apart?
Unlike other blood cancers, MPNs and MDSs are clonally driven disorders, meaning they start with a single rogue blood-forming cell that divides more than it should. That process is called clonal hematopoiesis, and it’s often fueled by long-term inflammation in the body.
This connection to inflammation may help explain why Singh’s team saw a drop in MPN and MDS rates among patients taking GLP-1 receptor agonists. These drugs are known to lower inflammation — and some, like semaglutide and tirzepatide, stay in the body for several days at a time. That long-lasting effect may keep inflammation levels down consistently enough to shrink or slow the growth of harmful blood cell clones.
“Clonal hematopoiesis is associated with inflammation, and those clones thrive under inflammation,” Singh explains. “So, if you’re inhibiting something — with, let’s say, the GLP-1 drug’s inflammation inhibition — you’re probably modulating the clone size.”
Earlier GLP-1 drugs, like exenatide, had shorter half-lives, meaning their anti-inflammatory effects didn’t last long. But newer, longer-acting drugs may provide what Singh calls a “constant state of anti-inflammation,” enough to quietly reshape how these diseases progress, at least in certain high-risk patients.
A cautionary tale—what this does and doesn’t mean
“This is population data and by no way or means this is causation data.” Singh cautions. Like any retrospective study, it may show associations but can’t prove cause and effect.
The real test will come with researchers zooming in from population level to controlled experimental models. “Until we can prove this in my mouse models and then we can prove this in prospective clinical studies… I think this cannot be used to reduce risk of blood cancer or any cancer.”
He’s also mindful of the drug’s limits and risks. GLP-1 drugs are not without side effects, including gastrointestinal symptoms and potential risks like pancreatitis. “They were not designed at this time for widespread prevention in otherwise healthy individuals,” Singh says. While “we may be heading that route,” for now, these drugs are for diabetes and obesity — not for cancer prevention.
Looking ahead: From population clues to clinical trials
These population-level clues are an exciting start, a strong foundation for a deeper dive into the underlying biology. “I’m excited and biased,” Dr. Singh confesses with a smile, “because I sleep, breathe, eat, clonal hematopoiesis.” His CHIP (clonal hematopoiesis of indeterminate potential) clinic is already following patients with early signs of clonal blood disorders. Providing therapy to these patients that could halt disease progression is his mission.
Singh and others researching and treating MPNs and MDSs are hoping that GLP-1RA can do for the world of oncology what it’s currently doing for the cardiovascular field. “The goal is that this opens the doors to biomarker guided repurposing of metabolic drugs in oncology,” Singh speculates.
A new angle for cancer prevention research
GLP-1 receptor agonists aren’t a cure-all and they may never be the cancer prevention drug for everyone. But Singh’s study underscores a compelling shift in oncology: instead of treating cancer after it arrives, researchers are asking how to intervene earlier, especially in diseases driven by inflammation and clonal evolution.
Singh sees this as the beginning of a larger scientific journey — one that could reshape how researchers think about prevention. “These are all provocative findings that need further evaluation,” he says.
Whether or not GLP-1 drugs end up playing a direct role in cancer prevention, they’ve helped shine a light on new questions worth asking. And sometimes, that’s where the biggest breakthroughs begin.
Written by: Amielle Moreno, PhD
Sources:
Ashruf, O. S., Hundal, J., Mushtaq, A., Kaelber, D. C., Anwer, F., & Singh, A. (2025). Hematologic Cancers Among Patients with Type 2 Diabetes Prescribed GLP-1 Receptor Agonists. JAMA Network Open, 8(3), e250802. https://doi.org/10.1001/jamanetworkopen.2025.0802
Anderer, S. (2025). FDA Accepts Application for Oral Version of Wegovy. JAMA, 334(1), 12. https://doi.org/10.1001/jama.2025.7761
Zhao, X., Wang, M., Wen, Z., Lu, Z., Cui, L., Fu, C., Xue, H., Liu, Y., & Zhang, Y. (2021). GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Frontiers in Endocrinology, 12. https://doi.org/10.3389/fendo.2021.721135


